The degradation of messenger RNAs (mRNAs) is an essential step in the regulation of gene expression. This mechanism plays a key role in the elimination of defective forms of mRNA as well as in the transient production of proteins in response to environmental changes. Degradation of mRNAs is triggered by the activation of cell signaling pathways that leads to the recruitment of destabilizing proteins and ribonucleases to the target mRNA. Destabilizing proteins are phosphorylated on multiple serine residues probably involved in the control of the assembly of these complexes. However, the impact of these phosphorylations on the activation or inhibition of mRNA degradation is not clearly defined. A better characterization of these mechanisms is necessary in order to better understand the impact of their dysregulation in human pathologies, particularly in cancer.
Some years ago, researchers from our laboratory have shown that TIS11b is a protein that destabilizes the mRNA encoding the Vascular Endothelial Growth Factor (VEGF). They also successfully developed an anti-tumor strategy based on the degradation of short-lived mRNAs by TIS11b in preclinical tumor models.
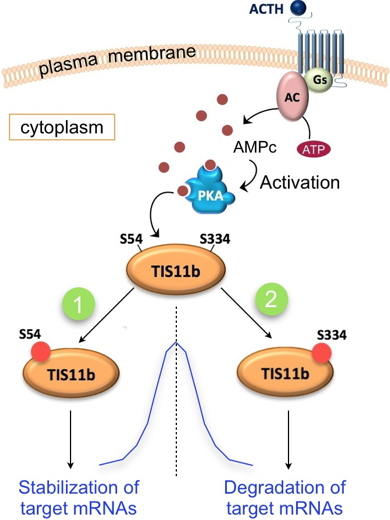
As part of recent studies, these researchers showed that in response to hormonal stimulation of endocrine cells, the TIS11b protein is phosphorylated by PKA (cAMP-dependent protein kinase) on two distal serine residues at the N-terminal and C-terminal ends of the protein (
Figure). These phosphorylations have antagonistic effects on the mRNA-destabilizing function of TIS11b. Dissection of the interactions between TIS11b and the mRNA degradation machinery in response to cAMP allowed the team to demonstrate for the first time that the C-terminal serine phosphorylation promotes the recruitment of mRNA-degrading enzymes. Researchers thus propose that the sequential phosphorylation of TIS11b on these two distal serine residues allows to stabilize then to degrade the target mRNA, thus leading to its transient expression (Figure).
These observations open new avenues for the development of therapeutic strategies targeting the assembly of mRNA degradation complexes in tumor cells.