 Head of the project
Head of the project Christel Marquette
Christel MarquettePreeclampsia is a specific complication of pregnancy characterized by hypertension, edema, high blood pressure and new-onset proteinuria, affecting 3-5% of all pregnancies. In preeclamptic pregnancy, placentation is incomplete, leading to the development of a hypoxic environment with soluble inflammatory factors released into the circulation by the stressed placenta. This leads to several systemic diseases such as thrombocytopenia, elevated liver enzymes, acute renal failure, pulmonary edema and cerebral dysfunction. The symptoms of pre-eclampsia are not limited to pregnancy, but continue well beyond. In fact, the risk of developing cardiovascular disease, hypertension and diabetes is increased 20 years after preeclampsia. Recently, several clinical studies have reported a threefold increased risk of vascular dementia and cognitive impairment in these women, with symptoms of anxiety, depression, memory and concentration problems. It is now well established that disturbances to the cerebrovascular system can be a prelude to the development of dementia and neurodegenerative diseases. In fact, in preeclampsia, changes in these brain functions appear to be associated with changes in the white matter of the frontal/parietal/temporal lobes and a reduction in cortical volume.
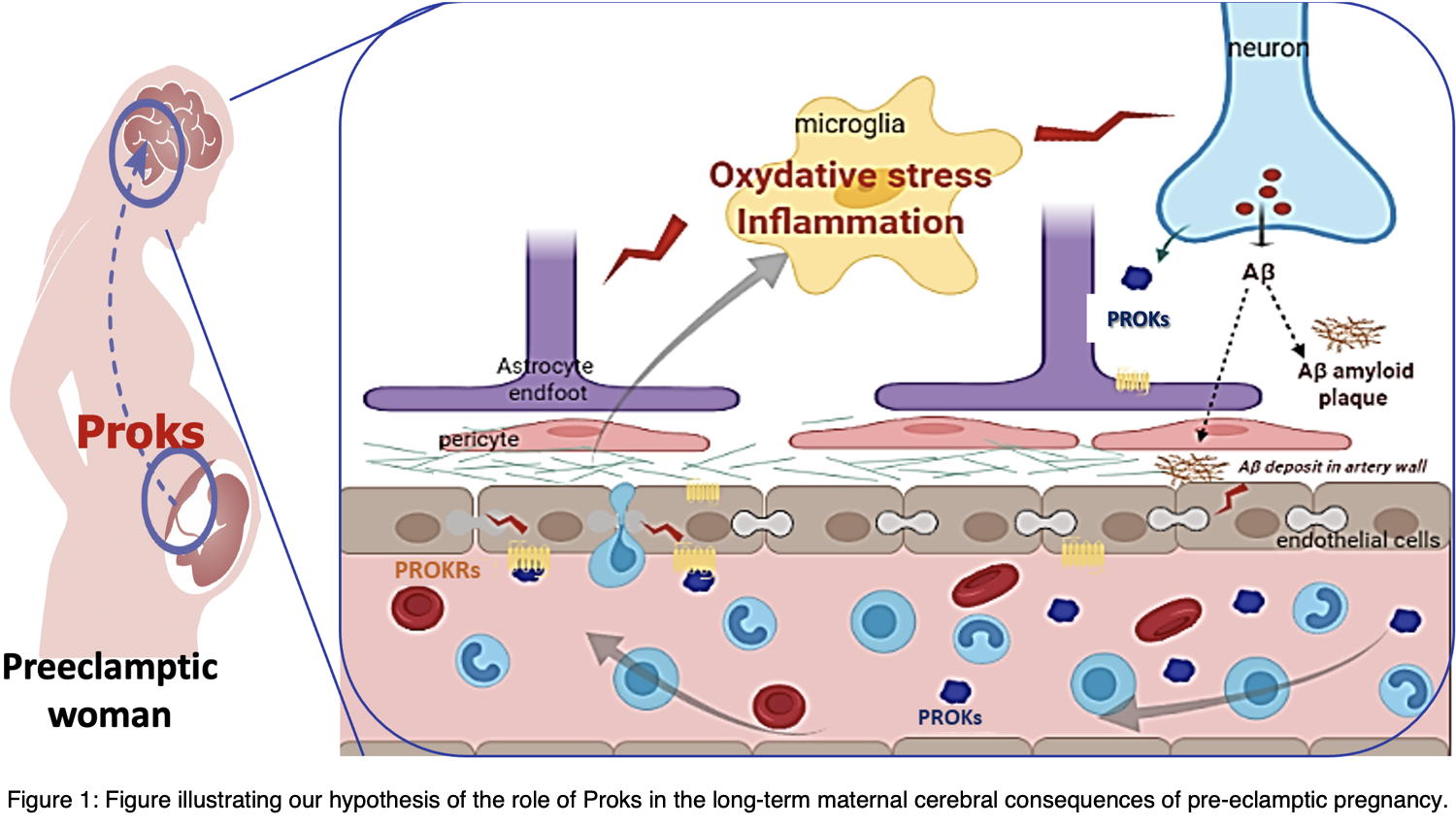
Dr. Christel Marquette's project aims to define whether and how these cerebral lesions are linked to circulating inflammatory factors released by the placenta during preeclampsia (Figure 1).
For several years, Dr. Nadia Alfaidy's team has been working on characterizing the prognostic and therapeutic value of members of the prokineticin family (PROKs and their receptors PROKRs) in pregnancy-related pathologies. These factors are involved in several physiological functions, including angiogenic and inflammatory processes, reproduction, the circadian cycle and neurogenesis. Recently, PROKs have also been implicated in neurodegenerative diseases such as Alzheimer's and Parkinson's, and in inflammation following stroke-induced brain damage. Previously, the team's investigations in preeclamptic women and in animal models of preeclampsia, showed that PROK factors are directly involved in the etiology of hypertensive preeclamptic pregnancies.
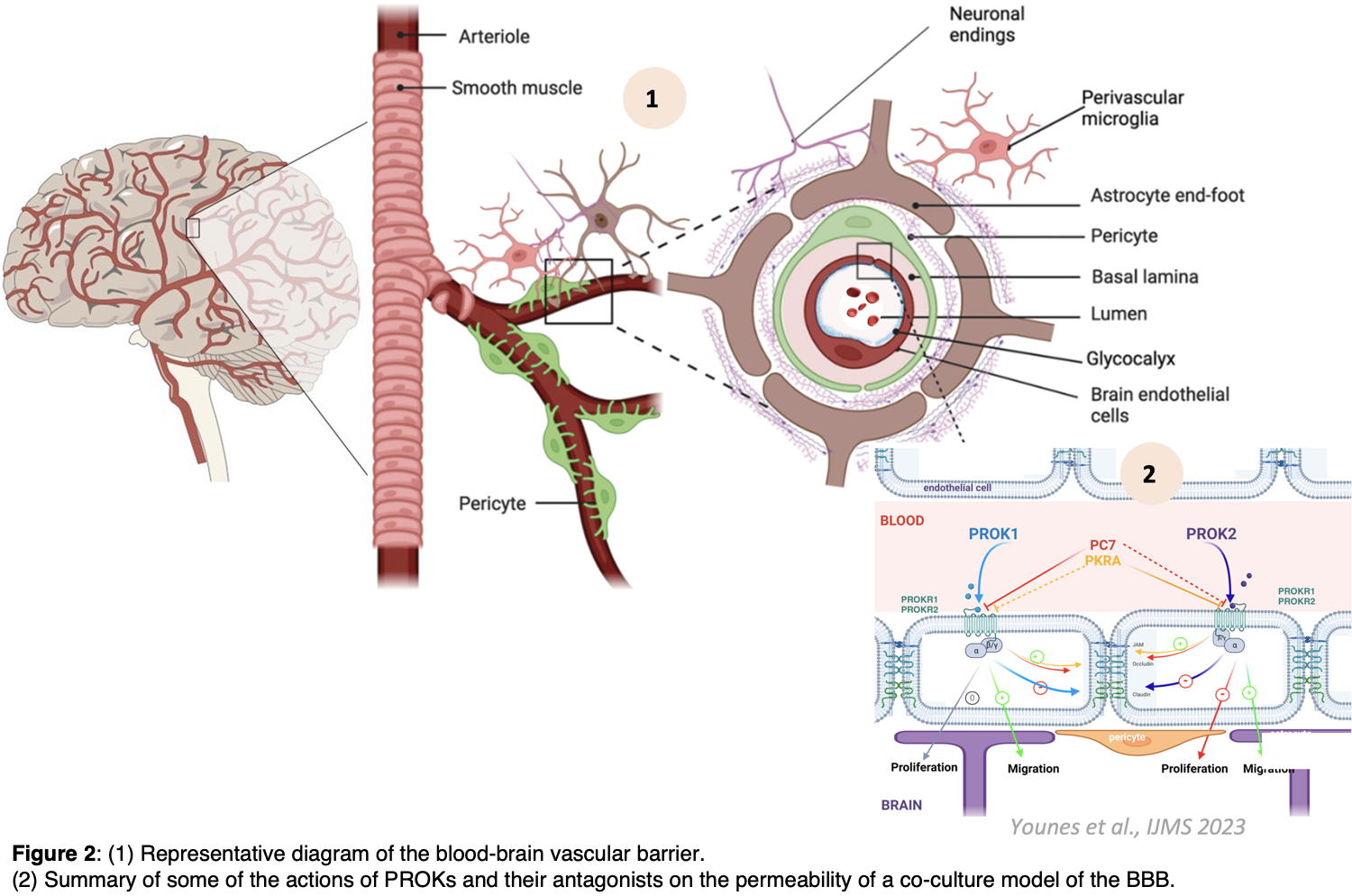
Our recent work in the preeclamptic mouse model established a direct link between the events of preeclampsia and subsequent maternal brain alterations. Furthermore, we have demonstrated the direct involvement of PROKs in the pathology of preeclampsia, as the treatment of mice during pregnancy with a PROKR antagonist significantly attenuated preeclampsia-mediated hypertension that is tightly associated with brain damage and inflammation. In addition, using a murine blood-brain barrier (BBB) configuration, we demonstrated that PROKs could specifically alter vascular permeability (Figure 2; PMID: 37895111).
Taken together, these original results demonstrate the direct link between preeclamptic events occurring during pregnancy and the onset of late cerebral alterations in women, and the major involvement of prokineticins in the short and long-term consequences observed latter on.
The aim of my research group is therefore to identify a preventive treatment that would alleviate the symptoms associated with preeclampsia during pregnancy, and prevent the onset of long-term cerebral sequelae in the mother
The implementation of my research project involves three approaches:  Cellular studies.
Cellular studies. Vascular integrity and cellular changes associated with preeclampsia are studied using a blood-brain barrier model. The effect of circulating inflammatory factors, obtained from mouse models or PE women's sera, on endothelial barrier’s integrity is tested through the characterization of the status of tight junction proteins and the BBB functionality (study of permeability and transport activity).
 In vivo and
ex vivo approaches
In vivo and
ex vivo approaches are implemented to characterize vascular and inflammatory changes using the brains of PE mice both at the time of the preeclamptic event during gestation and later on after delivery. This is achieved with pregnant mice treated or untreated with the prokineticin receptor antagonists, PC1 (a non-peptide antagonist preferring PROKR1) or PKRA (a non-peptide antagonist preferring PROKR2). The inflammatory state of the brain is assessed by deciphering tissue levels of inflammatory and redox factors, and complemented by immunohistochemical studies to determine reactive edema, vascular changes and reactive gliosis
 In vivo studies
In vivo studies (in collaboration with the Grenoble Neuroscience Institute). Brain imaging analyses (MRI) from PE-mice are performed to establish correlations between gestational hypertension and the onset of ischemic events. Cognitive disorders are also assessed using specific behavioral tests.
This project is meant to elucidate the underlying mechanisms affecting cerebral vascularization in the context of preeclamptic pregnancy, and the risk of patients developing dementia or even neurodegenerative pathologies. Moreover, our work provides insights into the potential use of PROKR antagonists as therapeutic options for the treatment of pre-eclampsia and its consequences.