Despite significant progress in conventional or targeted cancer therapies, therapeutic resistance remains a significant clinical challenge in the treatment of human malignancies. Multiple mechanisms are involved including selection of resistant cells during cancer progression and establishment of compensatory cellular signaling pathways that promote tumor growth. Could we overcome cancer resistance?
The control of messenger RNA (mRNA) stability/degradation is a major mechanism for the regulation of gene expression, which is carried out by specific mRNA-binding proteins (RBP) and/or microRNAs. Through their binding to regulatory sequences in the mRNA, RBP induce the recruitment of molecular complexes promoting mRNA stabilization or degradation. Deregulation of these mechanisms is a hallmark of cancer cells. Notably, a prolonged stabilization of some mRNAs leads to an overexpression of proteins promoting tumor progression and aggressiveness.
Researchers from our laboratory had shown in a previous study that intra-tumoral injection of the mRNA-binding protein called TIS11b/BRF1 in mouse lung cancer inhibits the formation of blood vessels that cause tumor growth and metastatic spread. Using genetic engineering, these researchers recently developed a second-generation protein of which the mRNA-destabilizing activity has been greatly improved. Injection of this modified protein into breast tumours in mice resulted in significant inhibition of tumour growth (
Figure) and a dramatic reduction in the expression of markers of tumour invasion, inflammation and metastatic spread.
This innovative multi-targeted anti-cancer strategy demonstrates that the simultaneous degradation of a set of mRNAs is a relevant approach to halt the expression of several genes that promote tumor progression and aggressiveness.
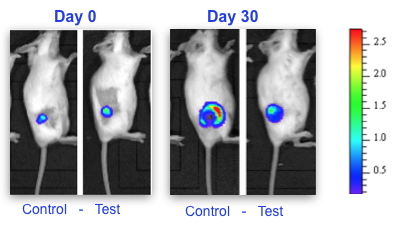
Measurement of tumor growth by bioluminescence imaging (IVIS system, Calipers) in mice showing breast tumors treated or not (Control) with the modified TIS11b protein (Test). Blue-red scale: increasing number of living tumour cells.