Kidney cancers are well known for their resistance to radio/chemo-therapeutic treatments as well as to targeted therapies against extensive neo-vascularization. This formation of new functional blood vessels (angiogenesis), is often the cause of resistance to treatment as it provides the irrigation and nutrient supply necessary for tumor growth. Many cancers originate from the aberrant activation of intracellular signaling pathways in which protein kinases play essential roles. Therefore, the search for new drugs targeting these enzymes may be a privileged approach within the framework of targeted therapies, aiming at disrupting the activity of some of these dysregulated enzymes.
Researchers in our laboratory intend to identify new candidate targets for the treatment of metastatic clear cell renal cell carcinoma (mccRCC), the eighth most common cancer in the world and representing 4% of all cancers. Based on the principle of
synthetic lethality, they evaluated the effects of more than 8,000 protein kinase inhibitor combinations on the viability of a cell line representative of metastatic renal cancer using a
chemogenomic screening. The screening performed in the Center for the screening for bio-active molecules in our laboratory, allowed the selection of one of these combinations targeting two protein kinases involved in cell survival and DNA repair mechanisms: protein kinases CK2 and ATM. For several years, IRIG researchers have shown that protein kinase CK2 is involved in multiple biological events such as cell plasticity, response to stress and cell proliferation. In addition, they have unambiguously demonstrated that this enzyme is dysregulated in many cancers (kidney, prostate, breast, choliocarcinoma...) fostering the development of an innovative approach to inhibit CK2 using small chemical molecules. The ATM protein kinase which is activated following DNA double-strand breaks is a key player in the DNA repair machinery.
In their study, the researchers found that simultaneous inhibition of the CK2 and ATM kinases by small chemical molecules in renal tumor cells and in patient tumor samples induces synthetic lethality. Importantly, this dual inhibition spares normal cells. Mechanistic studies carried out on renal carcinoma cells cultured as spheroids (3D culture) revealed that this double inhibition causes an excessive production of intracellular free radicals leading to massive cell death by apoptosis.
These results highlight the interest of a therapy combining the simultaneous inhibition of protein kinases CK2 and ATM to treat resistant/aggressive forms of kidney cancer.
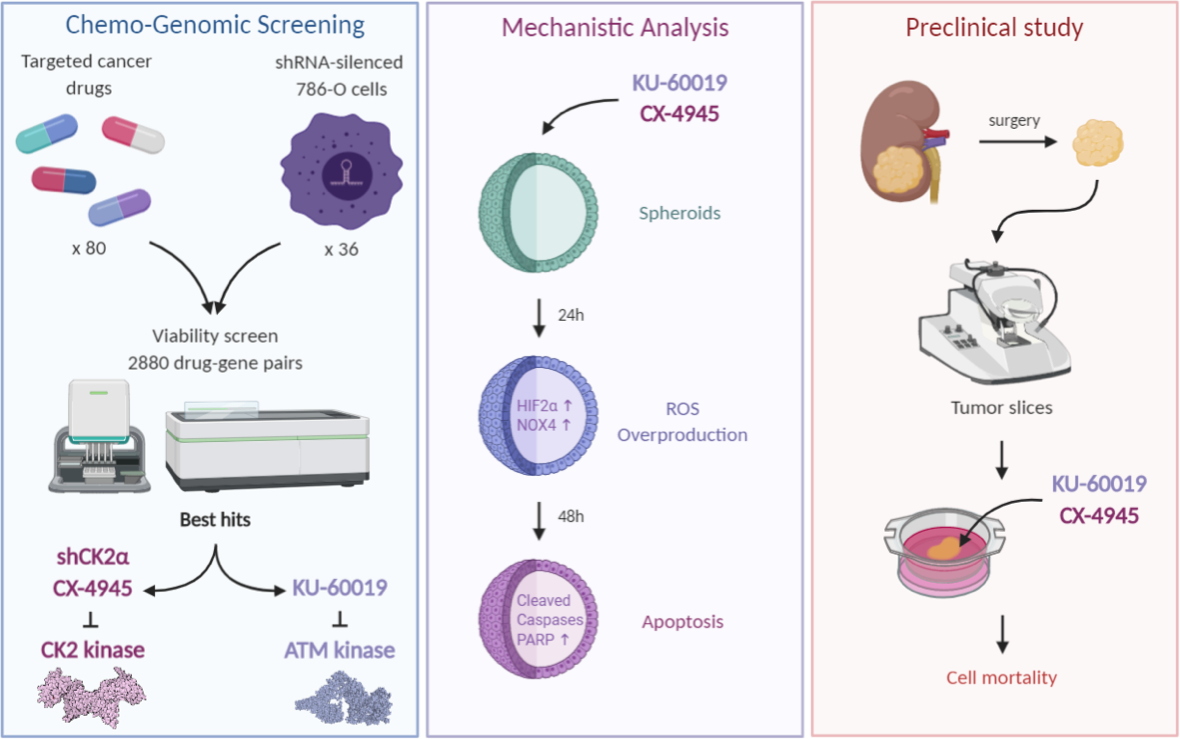
A. Chemogenomic screening: 80 small chemical molecules targeting protein kinases, tested on 36 786-O renal cancer cell lines, each expressing a shRNA to decrease the expression of a particular gene identified an inhibitor-shRNA couple (KU-60019-shCK2α) that is particularly effective in affecting cell viability. This couple targets the ATM and CK2 protein kinases, respectively. Then, shRNA directed against CK2α was replaced by a chemical molecule, CX-4945, which specifically inhibits this enzyme.
B. Analysis of the mechanism of action of the combination (KU-60019 and CX-4945) using spheroids (3-dimensional cell culture) shows that these inhibitory molecules induce,
via HIF-2α and NOX4 proteins, an overproduction of reactive oxygen species (ROS) leading to massive cell death.
C. Tested on human kidney tumor samples (COMBOREIN preclinical study), this combination of inhibitors shows its effectiveness in inducing cancer cell death.
Synthetic lethality: cell death resulting from the deficiency of two or more genes/proteins.
Chemogenomic screening aims to identify among chemical molecules and/or interfering RNAs that target proteins whose inactivation induces an interesting cell phenotype.