Copper is a trace element essential for cell function. However, high concentrations of this metal are cytotoxic as contribute to the production of reactive oxygen species responsible for damages to cellular components. It is now well established that the production and action of reactive oxygen species contribute to the development of diseases such as cancer, nervous system disorders, aging, but also to some pathologies of pregnancy. Indeed, high levels of copper and oxidative stress before conception or during pregnancy are associated with pre-eclampsia and intrauterine growth retardation (IUGR), two main pathologies of pregnancy responsible for a large number of premature births. To date, the mechanisms of the development of these diseases remain poorly understood.
In humans, copper homeostasis is ensured by various proteins that achieve its chelating, transfer between different cellular compartments, and transport. Among these proteins is the cellular prion protein (PrPC), a protein that binds copper
in vitro and
in vivo at the
N-terminal region, and thus contributes to its homeostasis. PrPC protein is ubiquitously expressed and it is well established that its altered conformational form is the causative agent of bovine spongiform encephalopathy. However, the physiological function of PrPC is poorly understood. Mice lacking PrPC are viable and do not have obvious phenotype.
In collaboration with the Chemistry and Biology of Metal Laboratory, we showed that, the protein PrPC is highly expressed during pregnancy compare to other proteins known to control copper levels in other systems
[1]. Interestingly, this expression is the highest during the first trimester of pregnancy, a key period for placental development and for the success of pregnancy. This suggests that copper homeostasis must be critical for the development of the placenta and the fetus.
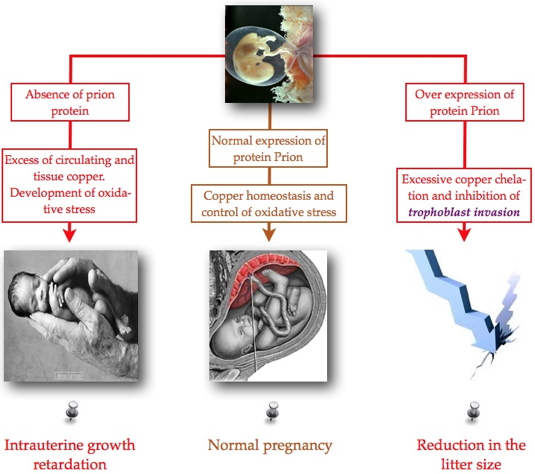
In the present study
[2], we investigated the role of PrPC during this critical period of development. At the cellular level, they demonstrated that PrPC plays a protective antioxidant role to insure different placental angiogenic processes (proliferation, migration, angiogenesis). This protective role of oxidative stress was further confirmed
in vivo. For the
in vivo study normal mice, mice that do not express the gene for PrPC (PrnP-/-) and mice that overexpress the protein (TGA20) were used. The three strain of mice have been treated or not by copper during their gestation. Our results showed that the loss of PrPC expression or it overexpression are respectively responsible for intrauterine growth retardation, or for a reduction in their litter size. These defects were correlated with structural and functional abnormalities in the placenta, as well as in a maintenance of abnormal
placental hypoxia beyond 10.5 days after conception.
This study demonstrated for the first time the physiological role of PrPC protein in placental development, and suggests that deregulation of its expression is strongly associated with the development of pathologies of pregnancy such as intrauterine growth retardation. PrPC deregulation also affected the main angiogenic processes necessary for the establishment of the maternal-fetal circulation and for the growth of the placenta.
Placental hypoxia: a physiological decrease in oxygenation of the placenta is observed up to 10.5 dpc (day after coitus).
During pregnancy, there is a
trophoblastic invasion of the spiral arteries that turn them into vessels with low pressure and high flow, ensuring the establishment of the maternal-fetal circulation.