Nlrp7 is the most mutated gene in recurrent complete hydatidiform mole (CHM), a benign abnormal pregnancy that progresses in 20% of cases to a very aggressive cancer, gestational choriocarcinoma (CC). In 2019, researchers at tour laboratory demonstrated
[1] that the NLRP7 protein, a product of the
Nlrp7 gene expression, was directly involved in the proliferation, invasion and differentiation of the normal trophoblast, a cell type that form the placenta. Nevertheless, the potential role of
Nlrp7 in the development and progression of the CC has not been investigated.
Researchers at our laboratory hypothesized
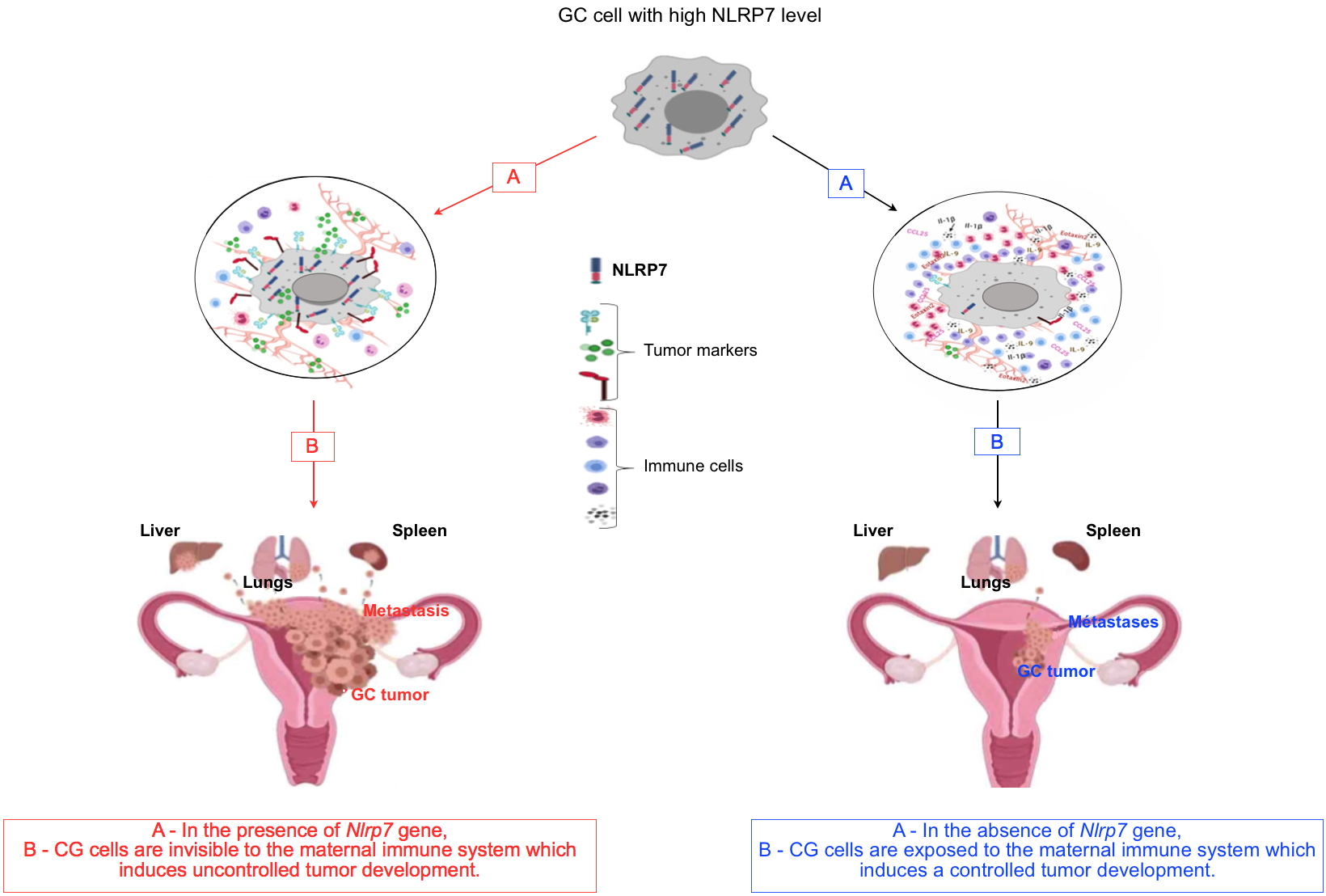
[2] that NLRP7 contributes to the acquisition of an immunosuppressive character by the placental tumor cell, which allows this cell to be camouflaged from the maternal immune system, to proliferate and metastasize to the brain, lung and liver. To demonstrate this hypothesis, they asked the following three questions:
Is the protein expressed at the same levels in the normal and tumor placenta? To answer this question, the researchers conducted a clinical study in close collaboration with the French center of Reference for Gestational Trophoblastic Disease and demonstrated that NLRP7 protein was significantly more expressed in the placenta of CHM or CC compared to placenta collected from normal pregnant women.
What would be the impact of Nlrp7 gene invalidation on the human placental tumor cell? To answer this question, the researchers invalidated the
Nlrp7 gene in the placenta tumor cell. They demonstrated that the absence of the NLRP7 protein directly affected the proliferation, invasion and three-dimensional organization of tumor cells. Also, they demonstrated that the absence of this protein alters the repertoire of immune tolerance markers of the tumor cell, exposing it further to the maternal immune control system.
Would a proof of concept involving an animal model of choriocarcinoma validate the clinical observations and the in vitro studies? To answer this question, the researchers used an animal model of choriocarcinoma that they have recently developed
[3]. This model is based on the injection of human tumor cells directly into the placenta of gravid mice with close monitoring of tumor development and progression. By comparing the tumor development in mice injected with tumor cells expressing or not the
Nlrp7 gene, they observed that the invalidated cells were more exposed to the maternal immune system. More importantly, mice injected with the
Nlrp7 knockout cells developed smaller tumors and had fewer metastases. Analysis of the tumor’s environment confirmed the increase in the activation of the pathways that control the maternal immune system.
Altogether, the findings of these researchers characterized the critical role of NLRP7 in the development of CC and highlighted its contribution to the establishment of an immunosuppressive maternal microenvironment that attenuates the maternal immune response while promoting the growth and progression of this cancer. The study proposes that NLRP7 plays a facilitating role in the proliferation of CC tumor cells and is therefore classified as a major player in the development of this cancer. These results strongly suggest that NLRP7 could be considered as a therapeutic target for the treatment of CC. Thanks to this discovery, the researchers, supported by the Regional League for Cancer, will be able to start clinical studies to confirm this hypothesis.