Protein-protein interactions form the interactome, a dynamic network with the ability to integrate both intra and extracellular signals and that plays a key role in the signalling pathways that regulate cell homeostasis. The structural and functional properties of these interactions and their deregulation in several different pathologies open up a range of new therapeutic opportunities.
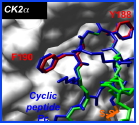 Protein kinase CK2 is a protein complex formed by the association of two catalytic subunits (CK2α) with two regulatory subunits (CK2ß). Crystallographic research paired with fluorescence imaging techniques have been used to determine that the formation of this complex within cells is a dynamic process whose functional significance needs to be evaluated. The imbalance in the expression of CK2 subunits, frequently observed in a variety of cancer-related diseases, highlights the flexibility of their interaction. The ability to interfere with the interactions that support formation of the CK2 complex would provide researchers with unique control over the key cellular events regulated by this enzyme. This research by researchers at our laboratory demonstrates that the CK2 multimeric structure can be reversed
in vitro. By coupling crystallographic analyses with directed mutagenesis, they have revealed that a limited number of CK2ß hydrophobic amino acids located at the interface control the affinity and act as interaction 'hot spots'. In particular, Tyr188 and Phe190 residues are major structural components of a CK2ß loop that fills a hydrophobic pocket on CK2α (Figure). These findings have enabled the rational design of small peptides capable of inhibiting this interaction. A constraint peptide formed of 11 amino acids has been proved to be particularly active in 1) blocking the association of the two subunits, 2) dissociating the CK2 complex and 3) altering its substrate specificity.
Protein kinase CK2 is a protein complex formed by the association of two catalytic subunits (CK2α) with two regulatory subunits (CK2ß). Crystallographic research paired with fluorescence imaging techniques have been used to determine that the formation of this complex within cells is a dynamic process whose functional significance needs to be evaluated. The imbalance in the expression of CK2 subunits, frequently observed in a variety of cancer-related diseases, highlights the flexibility of their interaction. The ability to interfere with the interactions that support formation of the CK2 complex would provide researchers with unique control over the key cellular events regulated by this enzyme. This research by researchers at our laboratory demonstrates that the CK2 multimeric structure can be reversed
in vitro. By coupling crystallographic analyses with directed mutagenesis, they have revealed that a limited number of CK2ß hydrophobic amino acids located at the interface control the affinity and act as interaction 'hot spots'. In particular, Tyr188 and Phe190 residues are major structural components of a CK2ß loop that fills a hydrophobic pocket on CK2α (Figure). These findings have enabled the rational design of small peptides capable of inhibiting this interaction. A constraint peptide formed of 11 amino acids has been proved to be particularly active in 1) blocking the association of the two subunits, 2) dissociating the CK2 complex and 3) altering its substrate specificity.
This peptide is the first antagonist to target the CK2 subunit interface, thereby inhibiting their interaction. The reseachers at currently using this peptide conformation as a support for the rational synthesis of chemical molecules capable of blocking this interaction.